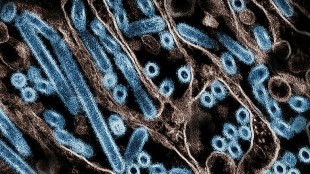
Ebola trial vaccines heading to Uganda: WHO
Three candidate vaccines against the strain of Ebola wreaking havoc in Uganda will be shipped to the East African country next week for trials, the World Health Organization said Wednesday.
Since Uganda declared an Ebola outbreak on September 20, cases have spread across the country, including to the capital Kampala, and have claimed 55 lives, with 22 more believed to have died.
Uganda has been struggling to rein in the outbreak caused by the Sudan strain of the virus, for which there is currently no vaccine.
But UN health agency chief Tedros Adhanom Ghebreyesus told reporters that vaccine trials would soon begin.
Speaking from the G20 summit in Indonesia, he said a WHO committee of external experts had evaluated candidate vaccines and determined "all three should be included in the planned trial in Uganda".
The WHO and the Ugandan health ministry accepted the committee's recommendation, he said, adding: "We expect the first doses of vaccine to be shipped to Uganda next week."
The WHO hailed the "incredibly fast collaboration" to reach this point.
"Since the outbreak began, the government of Uganda, together with researchers, funders, companies, regulatory authorities and other experts has been working under a global effort coordinated by WHO to accelerate the development and deployment of vaccines for use in trials," Tedros pointed out.
- 'Uncertainty' -
The candidates include a vaccine developed by Oxford University and the Jenner Institute in Britain, and another from the Sabin Vaccine Institute in the United States.
The third candidate came through the International AIDS Vaccine Initiative (IAVI), WHO said.
They will be used in a so-called ring vaccination trial, where all contacts of confirmed Ebola patients, and contacts of contacts are jabbed along with frontline and health workers.
"We have received written confirmation from the developers that sufficient vaccines and sufficient number of doses will be available for the clinical trial, and beyond if necessary," Ana Maria Henao Restrepo, one of WHO's heads of research and development, told reporters.
There is meanwhile concern that progress being made to slow the spread of Ebola even without the jabs could complicate the planned trials.
Such trials can only be run when there is fairly rapid transmission under way of Ebola, a haemorrhagic fever that spreads through close contact with bodily fluids and that is often deadly.
"We have uncertainty... about the evolution of the outbreak," Henao Restrepo said, acknowledging that it remained unclear "how many rings can be formed as part of the trial."
But she stressed that all those involved were committed to pushing ahead with randomised trials in a bid to "generate robust evidence that will allow us to know if one or more of them has the efficacy we hope they have."
- 'Controllable without vaccines' -
WHO emergencies director Michael Ryan insisted it was important to get started, even if lower transmission levels might make it impossible to complete the trials immediately.
"If we have to do this in one or two steps, we will," he told reporters.
At the same time, Ryan emphasised that "this epidemic is controllable without vaccines."
Experience from the far more frequent outbreaks of the Ebola Zaire strain -- for which a vaccine was developed after the massive West Africa outbreak that started in 2013 -- shows that "you can get control much quicker using effective vaccines," he told reporters.
"But just to reassure people in Uganda, we can stop this outbreak based on the current efforts."
Tedros agreed, insisting that Kampala's efforts had already "slowed transmission in most districts."
He pointed out that two districts had not reported any cases for 42 days, "indicating the virus is no longer present in those districts".
But he warned that Jinja had also reported its first case in the past week, "becoming the ninth district to be affected".
In addition to the candidate vaccines, the WHO said a separate group of experts had selected two investigational therapeutics for a trial, which still requires a green light from WHO and Ugandan authorities.
(R.Lavigne--LPdF)